Helminth eggs
Helminth eggs are the infective agents for the types of worm diseases known globally as helminthiases. Although helminths are pluricellular animals their eggs are microscopic (around 20 to 80 μm for those that are important in the sanitary field) and are contained in variable amounts in wastewater, sludge and excreta. Helminth eggs infect humans through: (1) the ingestion of food crops polluted with wastewater sludge or excreta, (2) direct contact with polluted sludge or faecal material, and (3) the ingestion of polluted meat or fish.
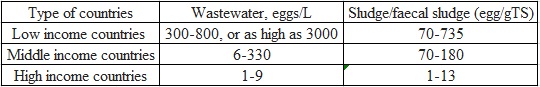
The eggs of different helminth species vary in terms of shape, size and resistance (figure 1). Due to different health conditions in different countries the type of helminth eggs and their content in wastewater and sludge also varies (Table 1). Ascaris eggs are the most common and ascariasis is the most common helminthiasis disease worldwide. The content of the other types of helminth eggs in wastewater determines the local patterns of disease.

a) Fertilised Ascaris b) Ascaris egg hatching c) Taenia egg, 30 - 40 mm
roundworm egg,
40-80 mm x 25-50 mm
Figure 1 Examples of helminth eggs found in wastewater, sludge or excreta. (a) and (c) from Atlas of Medical Parasitology, (b) courtesy of Catalina Maya, Treatment and Reuse GROUP, UNAM.
(TS: Total solids)
Table 1 Helminth ova content in wastewater and sludge from different income countries with data from: (Jiménez, 2008; Jiménez in press)
Eggs contained in wastewater, sludge or excreta are not always infectious. To be infectious they need to be viable and larval development needs to occur. This occurs after nearly 10 days of incubation at the required levels of temperature and moisture. These conditions frequently occur in soil or crops, where eggs are deposited when polluted wastewater, sludge or excreta are used as fertilizer.
Helminth eggs remain viable for 1-2 months in crops and for many months in soil, fresh water, and sewage. They may remain viable for several years in faeces, night soil and sludge (Feachem et al., 1989, Nelson et al., 2004; Sanguinetti et al., 2005; Koné et al., 2007). Because of this and because they are not inactivated - at least at affordably - using chlorine, UV-light or ozone they are considered highly resistant biological structures. This resistance derives from the composition of the egg shell. This consists of a variable number of layers (3-4 depending on the species) each providing mechanical resistance or protection from toxic compounds, such as strong acids, bases, oxidants, reducing agents, detergents and proteolytic substances. To inactivate helminth eggs it is recommended either to raise the temperature (above 40ºC), to reduce moisture (below 5%) or to maintain both of these conditions for an extended period of time. These conditions are not readily achieved during wastewater treatment, thus helminth eggs are usually physically removed from wastewater to be subsequently inactivated in sludge (Jiménez, 2008). In order to remove helminth eggs from wastewater, processes which remove particles, such as sedimentation, filtration or coagulation-flocculation are employed (Mara, 2004, Jiménez et al., 2010).
Not all treatment processes are efficient enough to inactivate the amount of eggs contained in sludge in developing countries, reliably or affordably (Carrington et al., 1991, Gantzer et al., 2001, Keller et al., 2004, Capizzi-Bananas, 2004; Jiménez and Wang, 2006; Koné, 2007).
WHO (2006) has published guidelines with regard to the level of treatment required. These suggest that a value of ≤ 1 egg/L in wastewater intended for the irrigation of crops to be eaten uncooked is safe. For aquaculture the proposal is that no eggs should remain at all, since trematode eggs (largely Schistosoma spp.) may multiply in an intermediary host (snails) before infecting fish and humans. For excreta, the recommended value is 1 egg/gTS.
There is no standardized analytical technique to detect and enumerate helminth eggs, and as a result few laboratories make these measurements. Those that do so employ different methods producing results that may be somewhat difficult to compare. Moreover, most of these laboratories only report the Ascaris content, and not the total concentration of helminth eggs. The most commonly used techniques are direct methods, in which the eggs are separated, recovered and concentrated from the sample, and are identified and counted under a microscope. Among these techniques are the membrane filter, Leeds I and II, Faust and US-EPA methods, all of which have different egg recovery rates (Ayres, 1989, Faust, 1939, US-EPA, 1992; Maya et al., 2006). Indirect techniques are based on measuring an alternative property, such as the total solids or particle content (Chavez et al., 2004) of the samples and relating these to the content of helminth eggs. To measure the viability of eggs, either incubation at 26ºC for 3-4 weeks to observe larval development or staining techniques (De Victorica and Galvan, 1993) are used.
References
Ayres R. (1989) A Practical Guide for the Enumeration of Intestinal Helminths in Raw Wastewater and Effluent from Stabilization Ponds. Leeds University Department of Civil Engineering.
Atlas of Medical Parasitology Carlo Denegri Foundation, downloaded on September 2010
Carrington E., Pike E., Autry D. and Morris R. (1991) Destruction of faecal bacteria, enteroviruses and ova of parasites in wastewater sludge by aerobic thermophilic and anaerobic mesophilic digestion. Water Science and Technology 24(2): 377-380.
Capizzi-Banas S., Deloge M., Remy M. and Schwartzbrod J. (2004) Liming as an advanced treatment for sludge sanitisation: helminth eggs elimination—Ascaris eggs as model. Water Research 38(14-15): 3251-3258
Chavez A., Jiménez B. and Maya C. (2004) Particle size distribution as a useful tool for microbial detection Water Science and Technology 50(2):179-186.
De Victorica, J. and Galvan, M. (2003) Preliminary testing of a rapid coupled methodology for quantization/viability determination of helminth eggs in raw and treated wastewater. Water Research 37(6):1278-87.
Faust E. C., Sawitz W., Tobie J., Odem V. and Peres C. (1939) Comparative efficiency of various techniques for the diagnosis of protozoa and helminths in faeces.Journal Parasitology 25: 241-262.
Feachem, R., Bradley, D., Garelick, H., Mara, D. (1983) Sanitation and Disease: Health Aspects of Excreta and Wastewater Management 349-356, New York, NY: John Wiley and Sons.
Gantzer C., Gaspard P., Gálvez L., Huyard A., Dumouthier N. and Schwartzbrod J. (2001) Monitoring of bacterial and parasitological contamination during various treatment of sludge. Water Research 35(16): 3763-3770.
Jiménez, B. (2008) Helminth Ova Control in Wastewater and Sludge for Agricultural Reuse. In: W.O.K. Grabow (ed.) Water reuse new paradigm towards integrated water resources management in Encyclopedia of Biological, Physiological and Health Sciences, Water and Health, Vol. II, Life Support System, pp 429-449. EOLSS Publishers Co Ltd. UNESCO.
Jiménez B. and Wang L. (2006) Sludge Treatment and Management, Chapter 10, pp 237-292 in Municipal Wastewater Management in Developing Countries: Principles and Engineering, Ujang Z. and Henze M. Editors. International Water Association Publishing. London, U.K.
Jiménez, B., Mara D., Carr R. and Brissaud, F. (2010) Wastewater treatment for pathogen removal and nutrient recovery: Suitable systems for use in developing countries, Chapter 8 in: Wastewater Irrigation and Health: Assessing and Mitigating Risks In Low-Income Countries, Dreschel and Scott Editors, Earthscan Press. London, pp. 149-170
Jiménez B. (in press) Safe sanitation in low economic development area, Treatise MS 82. Treatise on Water Science Ed. Elsevier.
Keller R., Passamani F., Cassini S. and Goncalves F. (2004) Disinfection of sludge using lime stabilization and pasteurization in a small wastewater treatment plant.Water Science and Technology 50(1): 13-17.
Kone D., Cofie O., Zurbru C., Gallizzi K., Moser D., Drescher S., Strauss M. , (2007) Helminth eggs inactivation efficiency by faecal sludge dewatering and co-composting in tropical climates Water Resarch 41(19): 4397-4402
Maya C., Jiménez B. and Schwartzbord J. (2006) Comparison of Techniques for the Detection of Helminth Ova in drinking water and Wastewater. Water Environment Research 78(2): 118-124.
Mara D. (2004) Domestic Wastewater Treatment in Developing Countries Ed. Earth Scan, London, 293 pp
Nelson K, J-Cisneros B., Tchobanoglous G. and Darby J. (2004) Sludge accumulation, characteristics, and pathogen inactivation in four primary waste stabilization ponds in central Mexico. Water Research 38(1):111-127.
Sanguinetti G., Tortul C., . García M, Ferrer V., Montangero V. and Strauss M. (2005) Investigating helminth eggs and Salmonella sp. in stabilization ponds treating septage, Water Science & Technology 51 (12): 239–247
US-EPA. (1992) Control of Pathogens and Vector Attraction in Sewage Sludge EPA/625/R-92-004. Washington, D.C.
WHO (2006) Guidelines for the Safe use of Wastewater, Excreta and Greywater in Agriculture and Aquaculture. Vol.1, 2, 3 and 4. World Health Organization, Ed. Paris, France.
Resources
This article was written by Dra. Blanca Jiménez-Cisneros, Instituto de Ingeniería, UNAM
Related Publications
Waterborne Pathogens: Review for the Drinking Water Industry - Emmanuelle Guillot and Jean-Francois Loret
Publication Date: Nov 2009 - ISBN - 9781843391791
Disinfection By-Products and Human Health - Steve E Hrudey and Jeffrey WA Charrois
Publication Date: May 2012 - ISBN - 9781843395195