Physico-chemical Water Treatment Processes
Contaminated water contains particles of different sizes which can be classified as dissolved (< 0.08 μm), colloidal (0.08 - 1 µm), supracolloidal (> 100 - 100 mm) and settleable (> 100 µm) (1 and 2).
The type of treatment selected depends on the size of particles present in the wastewater. In practice, treatment efficiency also depends on particle size.
Solids of the size that are visible to the naked eye can be separated either by settling under the influence of gravity or by flotation, depending on the relative densities of solids and water. They may also be easily separated by filtration. However, very fine particles of a colloidal nature (called colloids, size < 1 µm) which have high stability are significant pollutants. The reason for this stability is that these particles have electrostatic surface charges of the same sign (usually negative). This means that repulsive forces are created between them, preventing their aggregation and subsequent settling. It has therefore proved impossible to separate them by settling or flotation. It is not possible to separate these solids by filtration because they pass through any filter. However, separation by physico-chemical treatments is possible.
Physico-chemical treatment of wastewater focuses primarily on the separation of colloidal particles. This is achieved through the addition of chemicals (called coagulants and flocculants). These change the physical state of the colloids allowing them to remain in an indefinitely stable form and therefore form into particles or flocs with settling properties (3, 4 and 5).
Stages of The Physico-Chemical Process
The physico-chemical process consists of coagulation, flocculation and sedimentation stages (Figure 1). However there may be configurations where all stages are carried out in the same unit (6, 7, 8, 9 and 10).
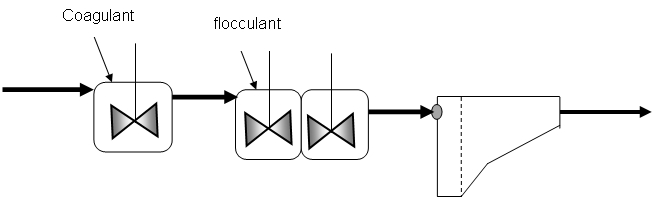
Figure 1. Components of conventional physico-chemical treatment (Adapted by reference 9).
Coagulation (or rapid mixing)
Derived from the Latin coagulare meaning driving together, coagulation refers to destabilization or neutralization of the negative charges contained in the wastewater by the addition of a coagulant applied during rapid mixing (which can vary from 250 - 1500 s-1) and a very short contact time (times ranging between 5 - 60 s) (4, 7, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 and 20). The quantity of coagulant applied during coagulation depends on the quality of water (domestic or industrial). In the case of domestic water, commonly used doses are < 50 mg/L, while for industrial water the dose is very variable.
The most commonly used coagulants are ferric chloride (11, 22, 23, 24, 25 and 26), ferric sulfate (24 and 27), aluminium sulfate (7, 14, 28, 29 and 30), aluminium polychloride (10, 13, 14, 16, 28 and 29), sodium aluminate mixtures of organic and inorganic compounds, lime and the more recently studied application of iron polychloride (21 and 31).
Flocculation (or slow mixing)
This is derived from Latin floculare, referring to the formation of flocs and bridges. In this stage, previously formed flocs group together, increasing in volume and density, allowing them to be sedimented. This is achieved by applying a gradient (10 to 100 s-1) and a contact time varying between 15 min and 3 h (3, 7, 11, 15, 16 and 17).
-By the movement of particles themselves (Brownian motion). In this case flocculation is referred to as perikinetic or natural convection (5, 10 and 32).
- By the movement of the fluid containing the particles, inducing their movement. This is achieved by agitating the mixture. This mechanism is referred to as orthokinetic or forced convection flocculation (3, 11, 16 and 18).
During the flocculation stage, chemicals referred to as flocculants are applied (assisted flocculation). These products allow flocs to come together and adhere, increasing their size and density. Flocculants can be classified by their nature (mineral or organic), their origin (synthetic or natural) or their electric charge (anionic, cationic or non-ionic).
Organic flocculants of natural origin are derived from natural products such as alginates (seaweed extract), starches (plant grain extracts) and cellulose derivatives. Their effectiveness is relatively low.
Those of synthetic origin are long chain macromolecules, soluble in water, formed by the association of simple synthetic monomers, some of which have electric charges or ionisable groups. For these reasons they are referred to as polyelectrolytes. These products are highly efficient and recommended concentrations are 0.05% -0.1% for solid products, 0.1% - 0.2% for liquid dispersion and 0.5% - 1.0% for liquids in solution. Applied in excess they may harm the flocculation process (5, 14, 15, 18, 28, 32, 33, 34, 35 and 36).
Sedimentation
This is the stage of floc removal by solid - liquid separation. For this, low, medium and high rate settlers are commonly used (17, 20, 34, 37 and 38). The rate is determined by the speed at which water and sludge are produced by the system.
Determination of Design and Operating Conditions Using "Jar Tests"
There are many aspects (physico-chemical properties of the wastewater of interest) that affect the performance of physico-chemical treatment. These can be determined by traditional laboratory jar tests (7, 9, 18, 22, 32, 40, 41 and 42) or by RoboJar systems (39). The system consists of six jars of the same size to which varying doses of coagulant are added at the same time. The system implements a rapid mixing sequence for a predetermined time followed by slow mixing for a set time and finally a settling sequence. After this, the supernatant is drained. Jar tests give a good approximation of the actual treatment process and the rapid mixing, slow mixing and sedimentation conditions of a real plant.
At the beginning, middle and end of the treatment tests, it is necessary to evaluate the efficiency of the process. This is achieved by measuring traditional parameters such as TSS, COD, pH, conductivity, turbidity, alkalinity, BOD, nutrients (N and P), thus establishing the efficiency of the system. Other parameters of greater accuracy such as particle size distribution (13, 15, 30, 43, 44 and 58), zeta potential and/or electrophoretic mobility (32) may also be used.
Applications
Physico-chemical treatment may constitute a single stage in the wastewater treatment process or be added as an additional treatment process during pre-treatment (to improve the biodegradation of wastewater in the biological process and secondary treatment (such as polishing).
Physico-chemical processes have been implemented for over 100 years (45). However in 1930, these processes were replaced by biological processes due to the high costs incurred by the treatment of large quantities of sludge (46). Recently, they have been reintroduced for various purposes: the elimination of phosphorus (17, 44, 45 and 47) for effluent being discharged to the sea, obtaining average quality effluent at lower cost than conventional treatments and for water used for agricultural irrigation (9, 20, 33 and 34), for potabilization (10 and 49), industrial water treatment (24 and 50) conditioning of sludge (primary and/or secondary) (23, 26, 27, 51 and 52). The resurgence of these processes is also due to increased recognition that the cost of treatment should be consistent with the desired efficiency, as progress in the synthesis of flocculation polymers with high efficiencies has been achieved at a lower cost (33).
Using this type of process it is feasible to remove 80 to 90% of total suspended solids (TSS), 40 to 70% of BOD5, 30 to 40% of COD and 17 to 100% of nutrients (N and P), depending on the dose and type of coagulant used (2, 7, 9, 17, 26, 33, 34, 47, 53 and 54). Heavy metals may also be removed by these processes, but the removal efficiency depends on the metal type and concentration (17, 33 and 55). Recently, these processes have been used to remove pathogens such as helminth eggs and have proven to be capable of removing up to 2 log concentration (20, 33, 34 and 48). In addition they are very efficient when used to remove bacteria (0-1 log unit), viruses and protozoa (1-3 log units in each case) (33 and 48). Current studies are focusing on their use for the removal of emerging contaminants (50, 56 and 57).
References
Levine A, Tchobanoglous G. and Asano T. (1991). Size distribution of particulate contaminants in wastewater and their impact on treatability, Wat. Res., 25(8): 911-922.
van Nieuwenhuijzen A.F, van der Graaf J., Kampschreur M. and Mels A. (2004). Particle related fractionation and characterisation of municipal wastewater, Wat Sci. and Tech., 50(12):125–132.
O´Melia Ch., Hahn M. and Chen Ch. (1997). Some effects of particle size in separation processes involving colloids,Wat Sci. Tech., 36 (4):119-126.
Menezes F.M., Areal R. and Luketina D. (1996) Removal of particles using coagulation and flocculation in a dynamic Separator, Powder Technology, (88):27-31.
Tambo N. and Watanabe Y. (1984). Physical aspect of flocculation process –III. Flocculation process in a continuous flow flocculation with back-mix floc, Wat. Res., 8(6): 695-707.
Rzbhun M, Fuhrer Z. and Adin A. (1984). Contact flocculation – filtration of humic substances. Wat. Res. 18(8): 963-970.
Amuda O.S and Alade A. (2006). Coagulation/flocculation process in the treatment of abattoir wastewater, Desalination, (196), 22–31.
Rossini M., Garcia G., and Galluzo M.(1999). Optimization of the coagulation –flocculation treatment: influence of rapid mix parameters, Wat. Res.. 33(8): 1817-1826.
Jiménez, B. and Chávez, A. (1997). Treatment of Mexico City Wastewater for Irrigation Purposes, Environmental Technology, 18(7): 721-729.
Bolto B. Gregory J. (2007) Organic polyelectrolytes in water treatment, Water Research, (41): 2301 – 2324.
Rattanakawin Ch. (2005). Aggregate size distributions in sweep flocculation. J. Sci. Technol., 27(5): 1095-1101.
Mhaisalkar V.A., Paramasivam R. and Bhole (1991). Optimizing physical parameters of rapid mix design for coagulation-flocculation of turbid water. War. Res. 25 (I): 43-52.
Chavez A., Jimenez B. and Maya C. (2004). Particle size distribution as a useful tool for microbialdetection Wat. Sci. and Tech., 50 (2): 179-186.
Kim S., Kim H., Moon B., Seo G. and Yoon C. (2006). Effects of addition sequence and rapid mixing conditions on use of dual coagulants. Wat. Sci. & Tech., 53(7): 87–94.
Lawler D. (1997). Particle size distribution in treatment processes: Theory and practice. Wat. Sci. Tech., 36(4):15-23.
Duan J and Gregory J. (2003). Coagulation by hydrolyzing metal salts. Advances in Colloid and Interface Science, (100 –102): 475–502.
Odegaard H. (1998). Optimised particle separation in the primary step of wastewater treatment. Wat. Sci. Tech., 37(10):43-53
Suarez S, Lema J., Omil F. (2009). Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresource Technology, (100): 2138–2146.
Park S, Jun H, Jung M and Koo H (2006) Effects of velocity gradient and mixing time on particle growth in a rapid mixing tank. Wat. Sci. & Tech., 53 (7): 95–102.
Jimenez, B. and Chavez, A. (1998). Removal of Helminth eggs in an advanced primary Treatment with sludge blanket, Env. Tech., 19 (11): 1061 – 1071.
Shao Y. Lui A., Wada F., Crosse J. and Jenkins D. (1996). Advanced primary treatment: An alternative to biological secondary treatment. The City of Los Angeles Hyperion treatment plant experience., Wat. Sci. Tech. 34 (3-4): 223-233.
Haarhoff J. and Joubert H. (1997). Determination of aggregates and breakup constants during flocculation. Wat. Sci. Tech. 35(4):33-40.
Amuda O. Amoo I. (2007). Coagulation/flocculation process and sludge conditioning in beverage industrial wastewater treatment, Journal and hazardous Materials, (141):778-783.
Ginos A., Manios T. and Mantzavinos D. (2006). Treatment of olive mill effluents by coagulation-flocculation-hydrogen peroxide oxidation and effect on phytotoxicity, Journal of Hazardous Materials, (B133): 135-142.
Poon C. and Chu C. (1999). The use of ferric chloride and anionic polymer in the chemically assisted primary sedimentation process. Chemosphere. 39(10):1573-1582.
Aguilar M. Sáez j., Lloréns M., Soler A., Ortuño J. (2002). Nutrient removal and sludge production in the coagulation-flocculation process. Wat. Res., (36): 2510-2519.
Aguilar M. Sáez j., Lloréns M., Soler A., Ortuño J. (2003) Microscopic observation of particle reduction in slaughterhouse wastewater by coagulation-flocculation using ferric sulphate as coagulants and different coagulants aids. Water Research, (37): 2233-2241.
Kan Ch., Huang Ch., Ruhsing J. (2002). Time requirement for rapid-mixing in coagulation. Colloids and Surfaces. A: Physicochemical and Engineering Aspect, (203): 1-9.
Tzoupanos N., Zouboulis A., Zhao Y. (2008). The application of novel coagulant reagent (polyaluminium silicate chloride) for the post-treatment of landfill leachates. Chemosphere (73): 729-736.
Adin A., Soffer Y. and Aim B. (1998). Effluent pretreatment by Iron coagulation applying various dose-pH combinations for optimum particle separation. Wat. Sci. Tech., 38(6): 27-34.
Liu T-K and Monica Ch-J. Improved coagulation performance using preformed polymeric iron chloride (PICl), Colloids and Surfaces A: Physicochemical and Engineering Aspect, (339): 192-198.
Tripathy T., Ranjan De B. (2006). Flocculation; a new way to treat the waste water. Journal of Physical Sciences. (10):93-127.
Jiménez B., Chávez A. and Hernández C. (1999): Alternative treatment for wastewater destined for agricultural use. Wat. Sci. Tech. 40(4-5):355-362.
Jimenez B., Chavez A., Leyva A. and Tchobanoglous G. (2000). Sand and synthetic medium filtration of advanced primary treatment effluent from Mexico City. Wat. Res. 34(2): 473-480.
Guibai L. and Gregory J. (1991) Flocculation and sedimentation of high-turbidity waters. Wat. Res., 25(9): 1137-1143.
Lee J., Liao P., Tseng D. and Wen P. (1998). Behavior of organic polymers in drinking water purification. Chemosphere, 37(6):1045-1061.
Lekang O., Bomo A and Svendsen I. (2001). Biological lamella sedimentation used for wastewater treatment. Aquacultural Engineering, (24):115-127.
Leung W-F and Probstein R. (1983). Lamella and tube settlers. Ind. Eng. Chem. Process Des. Dev., (22):58-67.
AWWA. ARF. (2007). Optimizing filtration processes through online floc particle characterization. Prepared by. Drewes J.. Ingels T. Yates G., Beierle A. 126 p.
Clark T. and Stephenson T. (1999). Development of a Jar testing protocol for chemical phosphorus removal in activated sludge using statistical experimental design. Wat. Res., 33(7) 1730-1734.
Marañón E., Castillón L., Fernández Y., Fernández A., Fernandez A. (2008). Coagulation-flocculation as a pretreatment process at a landfill leachate nitrification – denitrification plant. Journal of Hazardous Material, (156):538-544.
Ofir E., Oren Y. and Adin A. (2007). Comparing pretreatment by iron of electro-flocculation and chemical flocculation, Desalination, (204): 87-93.
Adin A. (1999). Particle characteristics: a key factor in effluent treatment and reuse. Wat. Sci. Tech. 40(4-5) 67-74.
Cripps S. (1995). Serial particle size fractionation and characterization of an aquacultural effluent. Aquaculture, (133):323-339.
Parker D., Bernard J., Gaigger G., Tekippe R. and Wahlber E. (2001). The future of chemically enhanced primary treatment: Evolution not revolution. IWACEPTREV3.doc. 17 p.
Harleman D. and Murcott S. (1999). The role of physical-chemical wastewater treatment in the mega-cities of the developing world. Wat. Sci. Tech., 40(4-5): 75-80.
Cripps S. (1995). Serial particle size fractionation and characterization of an aquacultural effluent. Aquaculture, 133:323-339.
Jimenez B., Chavez A., Maya C. and Jardines L. (2001). Removal of microorganism in different stages of wastewater treatment for Mexico City. Wat. Sci. Tech. 43(10): 155-162.
Lee J-F., Liao P-M., Tsen D-H and Wen P-T. (1998). Behavior of organic polymers in drinking water purification. Chemosphere, 37(6): 1045-1061
Goary F., Tawfik A. and Mahmoud U. (2010). Comparative study between chemical coagulation/precipitation (C/P) versus coagulation/dissolved air flotation (C/DAF) for pre-treatment of personal care products (CPCs) wastewater, Desalination, (252):106-112.
Kayode T. and Gregory J. (1988). A new technique for monitoring alum sludge conditioning. Wat. Res., 22(1):85-90.
Mahtab A., Tariq M., Shafiq T. and Nasir A. (2009). Coagulation/adsorption combined treatment of slaughterhouse wastewater, Desalination and Water Treatment, (12):270-275.
Jones P. (1973). Treatment in municipal plants; Innovations for removal of phosphorus. Wat. Res., (7):21-226.
Neis U. and Tiehm (1997). Particle size analysis in primary and secondary wastewater effluents. Wat. Sci. Tech., 36(4):151-158.
Landa H., Capella A. and Jimenez B. (1997) Particle size distribution in an effluent from an advanced primary treatment and its removal during filtration. Wat. Sci. Tech., 34(4): 159-165.
Carballa M. Omil F., and Lemma J. (2005). Removal of cosmetic ingredients and pharmaceuticals in sewage primary treatment. Wat. Res., (39):4790-4796.
Auriol M., Filali Y., Tyagi R. Adams C. surampalli R. (2006). Endocrine disrupting compound removal from wastewater, a new challenge. Process Biochemistry., (41): 525-539.
Related Publications
Sustainable Treatment and Reuse of Municipal Wastewater - Menahem Libhaber and Alvaro Orozco Jaramillo
Publication Date: Jun 2012 - ISBN - 9781780400167
Best Practice Guide on Metals Removal From Drinking Water By Treatment - Mustafa Ersoz and Lisa Barrott
Publication Date: Jun 2012 - ISBN - 9781843393849
Groundwater Set - Christian Kazner, Thomas Wintgens, Peter Dillon; Harvey Wood; C.G.E.M. (Kees) van Beek; Milan Dimkic, Heinz-Jurgen Brauch and Michael Kavanaugh; Philip E. LaMoreaux, et al.; M Brown, B Barley, H Wood
Publication Date: May 2012 - ISBN - 9781780404493
Disinfection By-Products and Human Health - Steve E Hrudey and Jeffrey WA Charrois
Publication Date: May 2012 - ISBN - 9781843395195
Water Reclamation Technologies for Safe Managed Aquifer Recharge - Christian Kazner, Thomas Wintgens, Peter Dillon
Publication Date: Apr 2012 - ISBN - 9781843393443
Water Quality Set - Beate Escher and Frederic Leusch; Bob Breach; World Health Organisation (WHO); Satinder Ahuja
Publication Date: Jan 2012 - ISBN - 9781780401201
Developing Better Indicators for Pathogen Presence in Sewage Sludge - Suresh D. Pillai, Ph.D.
Publication Date: Dec 2011 - ISBN - 9781780400129
Bioanalytical Tools in Water Quality Assessment - Beate Escher and Frederic Leusch
Publication Date: Dec 2011 - ISBN - 9781843393689